The release of glucocorticoids from the adrenal glands is primarily controlled by adrenocorticotrophic hormone (ACTH). ACTH is normally secreted by the anterior pituitary, which itself is influenced by corticotropin-releasing hormone (CRH). CRH is secreted by the hypothalamus.

Published: 23rd December 2021
By Emily Armstrong BVSc MRCVS
Veterinary Technical Manager
Dechra Veterinary Products

What is Canine Cushing’s syndrome?
Canine Cushing’s syndrome is the term used for a range of clinical syndromes displayed in the dog, caused by a chronic excess of glucocorticoid activity. The condition can also be known as hyperadrenocorticism (HAC/hac) and cushing’s disease, however recent specialist recommendation discourages the use of this terminology (European Society of Veterinary Endocrinology ALIVE Committee, 2021). It is named after the American neurosurgeon, Harvey Cushing, who first described the condition in humans.
Although considered a rarer condition in humans, for canine companions, Cushing’s syndrome is one of the most commonly diagnosed endocrinopathies (Herrtage and Ramsey, 2012) and can affect a dog’s health, vitality and appearance.
Herrtage and Ramsey (2012) Canine hyperadrenocorticism. In BSAVA Manual of Canine and Feline Endocrinology. 4th edn. Eds C.T. Mooney and M.E. Peterson. British Small Animal Veterinary Association 167-189
What causes Canine Cushing’s syndrome?
In order to best understand the pathophysiology involved in Cushing’s syndrome, it is first important to understand the normal physiology of glucocorticoid regulation.
Normal Glucocorticoid Regulation add
Causes of Cushing’s syndrome add
Canine Cushing’s syndrome can be classified as naturally occurring or iatrogenic. Dogs with the naturally occurring form can be further classified as having either ACTH-dependent or ACTH-independent forms.
ACTH-Dependent Cushing’s Syndrome add
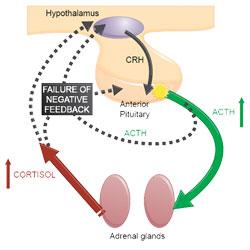
Most cases of naturally occurring Cushing’s syndrome (80-85%) (Melian et al, 2010) are caused by hypersecretion of ACTH by a lesion in the pituitary gland (typically pituitary neoplasia or hyperplasia). This leads to bilateral adrenal hyperplasia and increased glucocorticoid concentration in the blood. Excessive glucocorticoid activity due to cortisol is known as hypercortisolism, hence this form of Cushing’s syndrome is titled pituitary-dependent hypercortisolism (PDH).
Dogs with PDH have a failure of the normal negative feedback mechanism which prevent excess circulating cortisol. This is demonstrated in Figure 1.
Other, rarer forms of ACTH-dependent hypercortisolism include ectopic ACTH (where excessive ACTH is produced from a site away from the pituitary gland) and subdiagnostic Cushing’s syndrome (where clinical symptoms of Cushing’s syndrome are displayed, and excessive ACTH secretion is demonstrated, yet subsequent increases in cortisol cannot be proven).
ACTH-Independent Cushing’s Syndrome add
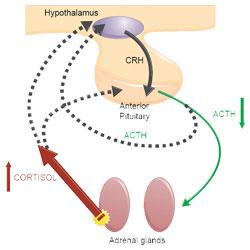
15-20% of cases of naturally occurring Cushing’s syndrome(Melian et al, 2010) are due to hypercortisolism because of unregulated cortisol secretion by the adrenal cortex, generally associated with adrenal neoplasia. This is more commonly known as adrenal-dependent hypercortisolism (ADH).
The increased serum cortisol concentration results in suppression of pituitary ACTH secretion and subsequent atrophy of non-tumorous adrenocortical tissue. This is demonstrated in Figure 2.
Other, rarer forms of ACTH-independent hypercortisolism include aberrant adrenal receptor expression (this includes food-dependent hypercortisolism) and subdiagnostic Cushing’s syndrome (where clinical symptoms of Cushing’s syndrome are displayed due to secretion by an adrenal tumour of a non-cortisol hormone that has glucocorticoid activity).
Iatrogenic Cushing’s Syndrome add
This form of Cushing’s syndrome is caused by chronic administration of systemic or topical exogenous glucocorticoids (for example this form of Cushing’s syndrome may be seen in dogs on long term therapy with prednisolone, or when steroid containing otic medication is used for prolonged periods)
What are the clinical signs of Cushing’s syndrome in dogs?
The clinical signs of Cushing’s syndrome can be categorised by how frequently they are identified at the time of initial presentation of the patient to the clinic (Behrend et al, 2013)
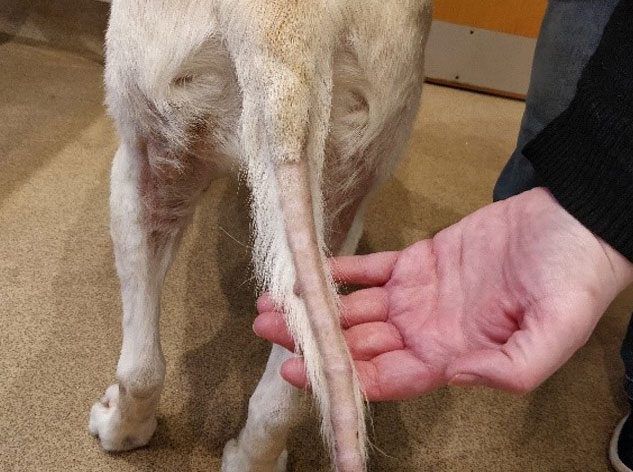
add
The image above shows a typical ‘rat tail’ which can be seen with alopecia of endocrine origin.
- Polydipsia (increased thirst)
- Polyuria (increased frequency of urination)
- Polyphagia (increased appetite)
- Panting
- Abdominal distention (otherwise known as a ‘pot belly’ and sometimes confused with weight gain or fluid retention)
- Alopecia (hair loss - mainly seen on the flanks and extremities.) Note that atopic dermatitis /pruritic conditions are NOT commonly associated with such an alopecia. The elevated production of glucocorticoids from the adrenal cortex as part of this disorder ‘mask’ any itch)
- Hepatomegaly (enlargement of the liver)
- Muscle weakness
- Systemic hypertension (increased blood pressure)
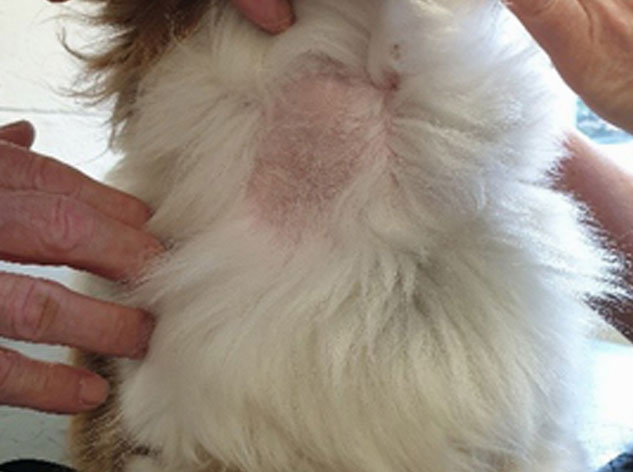
add
The image above shows lack of hair regrowth some time after blood sampling.
- Lethargy (or fatigue)
- Hyperpigmentation
- Comedones (‘black heads’)
- Thin skin
- Poor hair regrowth (after clipping for a blood sample or a groom for example)
- Urine leakage
- Insulin resistant diabetes mellitus
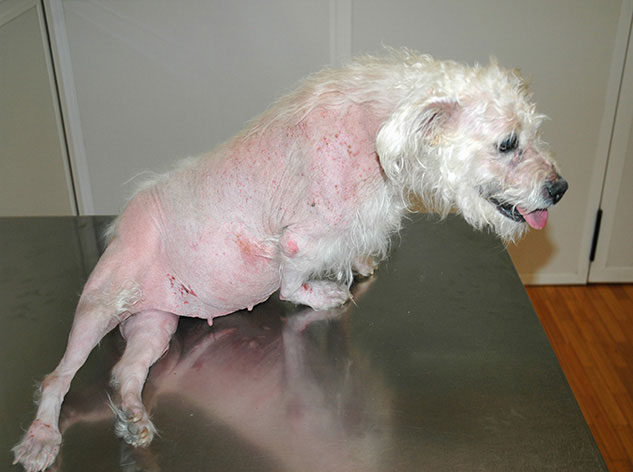
add
The image above (© Carlos Melina) shows a dog with pseudomyotonia.
- Thromboembolism (blood clot)
- Ligament rupture
- Facial nerve palsy
- Pseudomyotonia
- Testicular atrophy (entire male dogs) / Persistent anaestrus (entire female dogs)
As an aid memoire, Dechra recommend that clinicians think Cushing’s syndrome and think about the letter ‘P’, as many of the symptoms can be associated with the letter. Further explanation of this can be found in the video below:
Presence of any combination of the clinical signs listed above should increase suspicion of Cushing’s syndrome and should prompt initial non-specific laboratory testing, namely routine haematology, biochemistry and urinalysis.
The common laboratory abnormalities seen in cases of canine hypercortisolism are displayed in the table below:
Common laboratory abnormalities in dogs with hypercortisolism (adapted from Behrend et al. 2013)
Haematology
Increased neutrophils and platelets
Decreased lymphocytes and eosinophils
Mild erythrocytosis
Biochemistry
Increased alkaline phosphatase (ALKP) and alanine aminotransferase (ALT)
Hypercholesterolemia
Hypertriglyceridemia
Hyperglycaemia
Urinalysis
Low specific gravity ≤ 1.018 – 1.020
Proteinuria
Indicators of urinary tract infection
The combination of signalment, clinical signs and routine laboratory findings will then often lead to a presumptive diagnosis of Cushing’s syndrome. However, specific endocrine testing is then required to confirm diagnosis.
How is Canine Cushing’s disease diagnosed?
1. What is the best test to use to diagnose Cushing’s syndrome? add
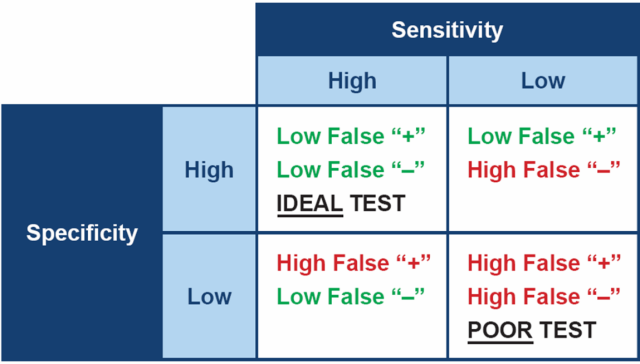
Ideally, when testing for any disease, it is best to choose a test which is both highly specific and highly sensitive. In practical terms, a test with high sensitivity is good at finding animals with the disease and therefore will produce few false negative results. A test with high specificity gives a positive result in animals that really do have the disease, therefore will produce few false positives.
However, no test for Cushing’s has both high sensitivity and high specificity – all are a compromise between the two. This means the diagnostic tests we have for canine hypercortisolism often have to be used in combination, in order to be confident in the diagnosis.
(Please note - the percentage values provided for sensitivity and specificity below should be considered approximate and are representative of multiple investigative studies on this topic. The author refers the reader to the 2019 Bennaim et al review for further information)
ACTH Stimulation Test (ACTHST):
High specificity (90%), moderate sensitivity (85% PDH and 50% ADH)
False positives are less common. False negatives are fairly common.
Low-Dose Dexamethasone Suppression Test (LDDST):
Moderate specificity (70%), high sensitivity (95%).
False positives are fairly common. False negatives are less common.
Urine Cortisol: Creatinine Ratio (UCCR):
Poor specificity (20%), high sensitivity (97%).
False positives are common. False negatives are less common.
When interpreting any of these tests, it is worth considering the positive and negative predictive value. Positive predictive value (PPV) is the fraction of dogs with a positive test that truly have Cushing’s. Negative predictive value (NPV) is the fraction of dogs with a negative test that are truly free of the condition.
The positive and negative predictive values take both the test and the population being tested into account. If you use a test in two populations with different disease prevalence, the predictive values will be different. Therefore the PPV and the NPV vary depend on how frequently you test.
When interpreting any of these tests, it is worth considering the positive and negative predictive value. Positive predictive value (PPV) is the fraction of dogs with a positive test that truly have Cushing’s. Negative predictive value (NPV) is the fraction of dogs with a negative test that are truly free of the condition.
The positive and negative predictive values take both the test and the population being tested into account. If you use a test in two populations with different disease prevalence, the predictive values will be different. Therefore the PPV and the NPV vary depend on how frequently you test.
- If we were to test almost every patient we see (a population with a low prevalence - 5%)
|
Test |
Positive predictive value |
Negative predictive value |
|---|---|---|
|
ACTHST (PDH) |
30% |
99% |
|
ACTHST (PDH) |
24% |
98% |
|
LDDST |
16% |
100% |
In this scenario, if we use the LDDST we can be 100% sure a negative result is truly negative. However only 16% of positive results will actually have Cushing’s.
- If we were more critical about the patients we test (a population with a high prevelance - 90%)
|
Test |
Positive predictive value |
Negative predictive value |
|---|---|---|
|
ACTHST (PDH) |
99% |
33% |
|
ACTHST (PDH) |
98% |
16% |
|
LDDST |
97% |
62% |
In this scenario, if we use the LDDST, 62% of negative results will truly be negative and 97% of positive results will have Cushing’s.
Conclusion
The 2012 ACVIM Consensus Statement panel (Behrend et al. 2013) considers the low-dose dexamethasone suppression test (LDDST) as the screening test of choice unless iatrogenic Cushing’s is suspected. Because of the ACTH stimulation test’s lower sensitivity its diagnostic usefulness as a screening test for natural occurring Cushing’s syndrome is inferior to the LDDST'.
However, it is also important to take other factors into account when selecting a diagnostic test, including test availability, cost and concurrent disease present in the patient. It is equally important to improve the potential outcome of the test by ensuring there is a high index of suspicion of disease before confirmatory testing is undertaken.
2. How do I undertake a low-dose dexamethasone suppression test (LDDST)? add
The low-dose dexamethasone suppression test measures the resistance of the pituitary-adrenal axis to suppression by dexamethasone. The following protocol has been prepared by Dechra in combination with specialist laboratories, however, if you have any queries, we would recommend checking this protocol with your regular laboratory before carrying out the test.
- Collect a basal fasted blood sample (1-2 ml) and label this tube as ‘pre Dex’. Suitable sample types at most laboratories include separated heparinised plasma or serum, or centrifuged serum gel tubes
- Immediately inject 0.01 mg/kg to 0.015 mg/kg dexamethasone I.V. It has been suggested that using 0.015 mg/kg dexamethasone may help reduce the chance of false positive results.
The volume of dexamethasone to administer in ml = (Bodyweight (kg) x Dose (mg/kg)) / Concentration of Dexamethasone solution (mg/ml). Volumes for injection are small for this test and, in some cases, it can be helpful to make a 1:10 dilution of dexamethasone before administration. - Collect two further blood samples, 3 hours and 8 hours post Dexamethasone injection. Label the sample times clearly on the tubes (e.g. ‘3hrs post’)
- Submit tubes and request form to the laboratory
In a normal healthy dog, the dexamethasone inhibits pituitary ACTH production via negative feedback, resulting in reduced cortisol production by the adrenal glands. Cortisol secretion is inhibited within 2 - 3 hrs and suppression lasts as long as 24 - 48 hr.
3. How do I interpret the results of a LDDST? add
The low-dose dexamethasone suppression test measures the resistance of the pituitary-adrenal axis to suppression by dexamethasone and is interpreted in 2 stages:
- Firstly, the presence or absence of Cushing’s syndrome is determined by examining the 8 hour result. An 8 hour cortisol value greater than 40 nmol/l is generally considered to represent a ‘positive’ result
Typically in a dog with ADH, the adrenal tumour secretes cortisol autonomously and ACTH production is already suppressed, thus cortisol production is not suppressed in response to dexamethasone administration.
- The second step only applies in the positive cases and checks for evidence of cortisol suppression. In up to 60% of PDH cases, there will be marked suppression of cortisol (to <50% of the baseline value) at either 3 hours or 8 hours providing a way to differentiate between the two types of hypercortisolism.
4. How do I undertake an ACTH Stimulation Test (ACTHST)? add
The ACTH stimulation test is a measure of adrenocortical reserve. The following protocol has been prepared by Dechra in combination with specialist laboratories and using the 2012 ACVIM consensus statement on diagnosis of spontaneous canine Cushing’s (Behrend et al, 2013). However, if you have any queries, we would recommend checking this protocol with your regular laboratory before carrying out the test.
- Collect a basal fasted blood sample (1-2 ml) and label this tube as ‘pre ACTH’ Suitable sample types at most laboratories include separated heparinised plasma or serum, or centrifuged serum gel tubes
- Immediately inject 5 µg/kg synthetic ACTH IV.
- Collect a second blood sample (1-2 ml) one-hour post injection of synthetic ACTH. Label tube as ‘post ACTH’
- Submit tubes and request form to the laboratory
The following video illustrates how a normal healthy dog should respond to an ACTHST. As you can see, the synthetic ACTH stimulates the adrenal glands to produce more cortisol, and subsequently levels should increase in the circulation. A normal increase is thought to be to around 300 – 400 nmol/l. A positive test result is normally defined as a 1 hour cortisol >600 nmol/l, in dogs with compatible clinical signs and no evidence of concurrent non-adrenal illness.
5. Can I get false negative results with the ACTHST? add
The ACTH stimulation test will identify approximately 85% of cases of pituitary-dependent hypercortisolism.
Dogs with PDH have bilaterally enlarged adrenal glands. With the greater adrenocortical mass, an exaggerated response to ACTH can be expected
The ACTHST will also identify >50% of cases of adrenal-dependent hypercortisolism, as most dogs with ADH will also demonstrate an exaggerated response to ACTH.
However, in some dogs, especially with ADH, there may be atrophy of the normal adrenal tissue and/or the tumour may be insensitive to ACTH. In these cases, as is demonstrated by this video, we can see a ‘flat-line, mid-range’ cortisol response. This explains why the ACTHST is less sensitive for ADH than PDH.
As the sensitivity of the test is less than ideal, especially for dogs with ADH, a diagnosis of Cushing’s should not be ruled out on the basis of a normal ACTH stimulation test result if there is sufficient clinical suspicion.
6. I have performed an ACTHST which has returned inconclusive – what should my next action be? add
The ACTH stimulation test will identify approximately 85% of cases of PDH and >50% of cases of ADH.
Therefore the sensitivity of the test is less than ideal, especially in ADH, and some dogs that actually do have hypercortisolism will have normal ACTH stimulation test results. Therefore a diagnosis of Cushing’s should not be ruled out on the basis of a normal ACTH stimulation test result if there is sufficient clinical suspicion.
In these circumstances it is recommended that a LDDST be undertaken in order to try to determine a correct diagnosis. Alternatively, if the clinical signs allow, the patient can be monitored clinically and the ACTHST repeated in 4-6 weeks’ time.
7. How do I reach a diagnosis of iatrogenic Cushing’s syndrome? add
The ACTHST is the only diagnostic test which allows us to differentiate between iatrogenic and spontaneously occurring Cushing’s syndrome. Chronic administration of exogenous glucocorticoid containing treatments, including oral and injectable glucocorticoid medications, topical ear drops and skin preparations, will cause suppression of the hypothalamic, pituitary adrenal axis. This, in turn, will produce a subnormal response to the ACTHST.
The following video illustrates this:
8. Do I need to differentiate between ADH and PDH once I have reached a diagnosis of Cushing’s syndrome? add
It is highly desirable to differentiate between pituitary-dependent hypercortisolism and adrenal-dependent hypercortisolism in order to provide a more accurate prognosis and enable the full range of possible treatments to be discussed with the dog’s owner.
Discriminatory tests available to differentiate between PDH and ADH include measurement of endogenous ACTH, the low- and high- dose dexamethasone suppression tests, ultrasonography and advanced imaging such as MRI and CT.
For further information on how Dechra can support veterinary surgeons and veterinary nurses with the diagnosis of Cushing’s syndrome, log-in to the diagnosis page.
The cushings syndrome diagnosis page contains a supportive range of resources to download, including a series of short webcasts with Dr Audrey Cook DACVIM DECVIM DABVP discussing the challenges of, and providing solutions to, communicating effectively with pet owners about diagnosis and treating cushings syndrome in dogs.
What are the treatment options for Cushing’s syndrome?
Treatment of Cushing’s may be achieved by three differing methods:
1. Surgery add
The surgical option for dogs diagnosed with pituitary-dependent hypercortisolism is transsphenoidal hypophysectomy. This involves the removal of the pituitary gland through the soft palate and can be curative if successful. As a specialist cranial surgery, transsphenoidal hypophysectomy is only offered at restricted veterinary hospitals throughout Europe, however it is increasing in popularity and availability.
The reported success rates for transsphenoidal hypophysectomy are good especially in those patients with smaller pituitary masses (<10 mm diameter) therefore patient selection and early decision making is important in these cases (Schofield et al 2021).
For patients with adrenal-dependent hypercortisolism, the surgical option is unilateral adrenalectomy to remove the primary adrenal mass. However, as many of the tumours involved in ADH are malignant, careful evaluation to ensure there is no metastatic spread is vital for success in these cases.
2. Pituitary irradiation add
Similarly to transsphenoidal hypophysectomy, pituitary radiation therapy is a specialist treatment modality and as such is only offered in a small number of facilities throughout Europe. This, combined with the need for multiple general anaesthetics (and associated costs) mean this treatment method is not widely utilised.
Regardless, it has been shown to reduce pituitary tumour size and is an option available for dogs with PDH.
3. Medical treatment add
Medical treatment of cushingoid dogs can be an excellent way to control the clinical symptoms associated with the syndrome.
Non-licensed medical therapies used to address Cushing’s syndrome include mitotane and ketoconazole. However trilostane is the only medication licensed for the treatment of pituitary-dependent and adrenal-dependent hyperadrenocorticism (Cushing's disease and syndrome) in dogs and is largely considered as a safe and effective drug for managing both PDH and ADH (Schofield et al 2021).
Further information on canine Cushing’s syndrome from Dechra
Dechra has created a dedicated treatment and monitoring page exclusively for veterinary professionals which contains a huge resource of additional content. This includes videos from Professor Stijn Niessen DVM PhD DipECVIM-CA MRCVS and downloadable monitoring documents created in collaboration with the Royal Veterinary College, London.
To access this exclusive content, veterinary professionals can log-in to receive access here:
Summary of key points
- Canine Cushing’s syndrome (previously referred to as hyperadrenocorticism or Cushing’s disease) is one of the most commonly diagnosed endocrine conditions in dogs
- It occurs as a result of chronically increased glucocorticoid concentrations in the blood (either produced naturally by the adrenal glands or administered exogenously to affected dogs)
- The two main types of naturally occurring Cushing’s syndrome diagnosed by veterinary practitioners are pituitary-dependent hypercortisolism (PDH) and adrenal-dependent hypercortisolism (ADH)
- Regardless of pathogenesis, dogs with Cushing’s syndrome display characteristic symptoms which include polyuria, polydipsia and polyphagia
- Diagnosis is made using a combination of clinical signs displayed and the findings from routine bloodwork, urinalysis and specific endocrine tests
- Aside from iatrogenic cases, there are no methods of prevention for Cushing’s syndrome
- Prognosis is good with appropriate treatment, of which medical and surgical options are available
Additional FAQ's
What is the impact of canine Cushing's syndrome? add
For the dog, Cushing’s disease can mean they develop a variety of clinical signs including drinking and urinating more, increased hunger and lethargy. For the owner, they must endure the impact of these symptoms on their daily life, which often results in both concern and frustration. Overall, Cushing’s can have a negative impact on quality of life for both the dog and the owner.
What happens if Cushing's is left untreated in dogs? add
If left untreated, symptoms are likely to progress over time. Dogs will continue to eat, drink and urinate more often. Lethargy can worsen, as can skin and coat changes associated with the syndrome. There is likely to be abdominal distention and eventually the quality of life of the affected dog can be seriously negatively impacted.
What is the life expectancy of a dog with Cushing's? add
When treated with trilostane (the main medical therapy for Cushing’s syndrome) it is anticipated that life expectancy of the patient is maintained. Importantly, however, it is expected that treatment with trilostane will improve quality of life for both the patient and owner alike.
What are the behaviour symptoms of Cushing's in a dog? add
The biggest behavioural changes that are seen with canine Cushing’s are related to eating, drinking and urination. Dogs may become ravenous and drink in excess. Equally, an increase in urination may often lead to accidents occurring within the house. Lethargy is also commonly noted - Cushingoid dogs may slow down on walks, may be unwilling to exercise and may spend more time sleeping.
What is the best treatment for Cushing's in dogs? add
There are two main forms of treatment for Cushing’s. The first is medical management, of which the licensed veterinary medication is trilostane. The second is surgical management which involves removal of tumour tissue from the adrenal gland or the from pituitary at the base of the brain. The option which is best will naturally vary from patient to patient, however Cushing’s is most commonly managed using medical therapy.
How long does it take for Cushing’s medicine to work? add
The main medical therapy for Cushing’s is trilostane and this must be given daily in order to control symptoms of disease. Owners will see results from trilostane treatment quickly – in as little as a few weeks improvement of clinical signs such as polydipsia, polyuria, polyphagia, panting and lethargy are expected.
Skin, coat and muscle changes may take a little longer to reverse but changes do not usually take longer than 3-6 months.
References add
-
Behrend et al (2013) Diagnosis of Spontaneous Canine Hyperadrenocorticism: 2012 ACVIM Consensus Statement (Small Animal) Journal of Veterinary Internal Medicine 1-13
-
Bennaim, M. et al (2019) Diagnosis of spontaneous hyperadrenocorticism in dogs. Part 2: Adrenal function testing and differentiating tests. The Veterinary Journal 252
-
Herrtage and Ramsey (2012) Canine hyperadrenocorticism. In BSAVA Manual of Canine and Feline Endocrinology. 4th edn. Eds C.T. Mooney and M.E. Peterson. British Small Animal Veterinary Association 167-189
-
Melian, C., Dolores Perez-Alenza, M. and Peterson, M.E. (2010) Hyperadrenocorticism in Dogs. In Textbook of Veterinary Internal Medicine. 7th Edn. Eds Ettinger, S.J. and Feldman, E.C. Saunders Elsevier 1816 – 1840
-
Niessen, S. (2021) European Society of Veterinary Endocrinology, Accessed 11th August 2021 https://www.esve.org/alive/search.aspx
-
Schofield, I. et al (2021) Update on the treatment options for canine hyperadrenocorticism In Practice 42(10) 540-546