-
Teng, K.T., McGreevy, P.D., Toribio, J.A.L., et al. (2018) Strong associations of nine-point body condition scoring with survival and lifespan in cats, Journal of Feline Medicine and Surgery, 20(12): 1110-1118
-
Freeman, L.M., Lachaud, M.P., Matthews, S., et al. (2016) Evaluation of weight loss over time in cats with chronic kidney disease, Journal of Veterinary Internal Medicine, 30(5): 1661-1666
-
Baez J.L., Michel K.E., Sorenmo, K., et al. (2007) A prospective investigation of the prevalence and prognostic significance of weight loss and changes in body condition in feline cancer patients, Journal of Feline Medicine and Surgery, 9(5): 411-417
-
Santiago, S.L., Freeman, L.M., and Rush, J.E. (2020) Cardiac cachexia in cats with congestive heart failure: Prevalence and clinical, laboratory, and survival findings, Journal of Veterinary Internal Medicine, 34(1): 35-44
-
Agnew, W., & Korman R. (2014) Pharmacological appetite stimulation: rational choices in the inappetent cat, Journal of Feline Medicine and Surgery, 16(9): 749-756
-
Chan, D.L. (2004) Nutritional requirements of the critically ill patient, Clinical Techniques in Small Animal Practice, 19: 1-5
-
Chan, D.L., & Freeman L.M. (2006) Nutrition in critical illness, Veterinary Clinics of North America: Small Animal Practice 36: 1225-1241, v-vi.
-
Freeman, L.M. (2012) Cachexia and sarcopenia: emerging syndromes of importance in dogs and cats, Journal of Veterinary Internal Medicine 26(1): 3-17
-
Corbee, R.J., & van Kerkhoven, W. (2012) Nutritional support for patients, Tijdschrift Diergeneesk, 137: 384-390.
-
Robben, J.H., et al. (1999) Enteral nutrition for the critically ill patient, Tijdschrift Diergeneesk, 124: 468-71.
-
Reynolds, C.A., Oyama, M.A., Rush, J.E., et al. (2010) Perceptions of quality of life and priorities of owners of cats with heart disease, Journal of Veterinary Internal Medicine, 24(6): 1421-1426
-
Tzannes, S., Hammond, M.F., Murphy, S., et al. (2008) Owners ‘perception of their cats’ quality of life during COP chemotherapy for lymphoma, Journal of Feline Medicine and Surgery, 10(1): 73-81
-
Bijsmans, E.S., Jepson, R.E., Syme, H.M., et al. (2016) Psychometric validation of a general health quality of life tool for cats used to compare healthy cats and cats with chronic kidney disease, Journal of Veterinary Internal Medicine, 30(1): 183-191
-
Armstrong, P.J., & Blanchard, G. (2009) Hepatic lipidosis in cats, Veterinary Clinics of North America: Small Animal Practice, 39(3): 599-616
-
Chan, D.L. (2009) The inappetent hospitalised cat: clinical approach to maximising nutritional support, Journal of feline medicine and surgery, 11(11): 925-933
-
Remillard, R.L. (2002) Nutritional support in critical care patients, Veterinary Clinics of North America: Small Animal Practice, 32(5): 1145
-
Cook, A.K. (2020) Top 5 indications for appetite stimulation, Clinician’s Brief, 31-35
-
Perez-Camargo, G. (2004) The aging feline: advances in nutrition and care for the older cat-cat nutrition: what is new in the old?, Compendium on Continuing Education for the Practicing Veterinarian, 26(2): 5-10
-
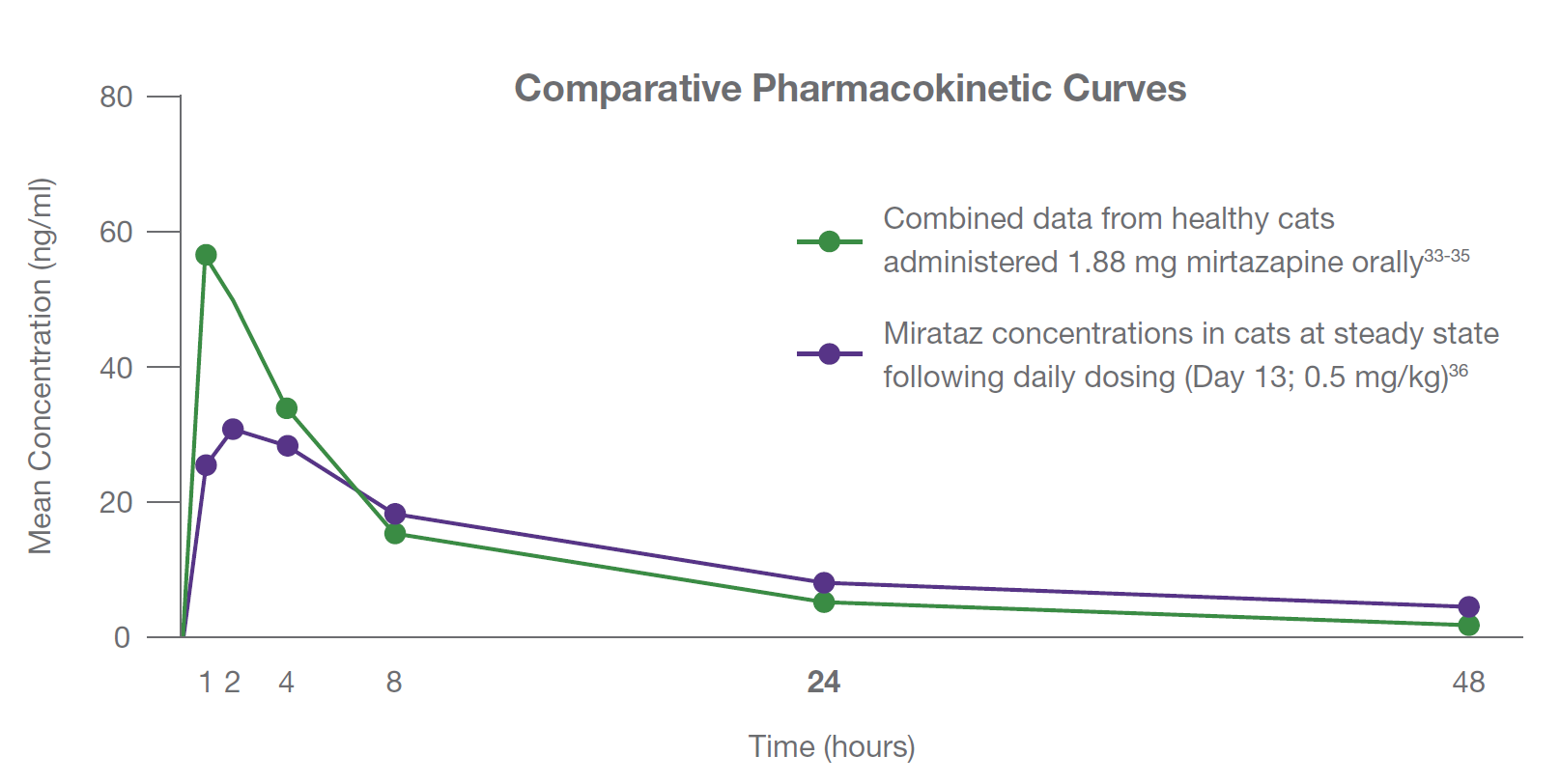
Dechra Internal report MZ-0272
-
Dechra Internal report MZ-2018
-
Pittari, J., Rodan, I., Beekman, G., et al. (2009) American association of feline practitioners. Senior care guidelines, Journal of Feline Medicine and Surgery, 11(9): 763-778
-
Laflamme D.,P. (2005) Nutrition for aging cats and dogs and the importance of body condition, Veterinary Clinics of North America: Small Animal Practice, 35(3): 713-742
-
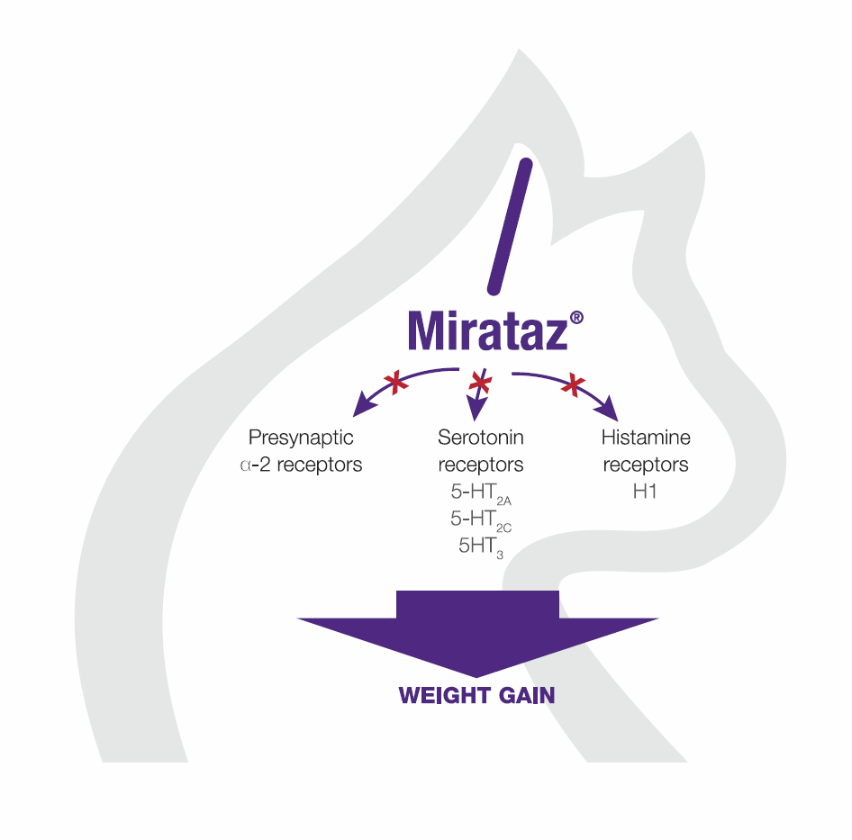
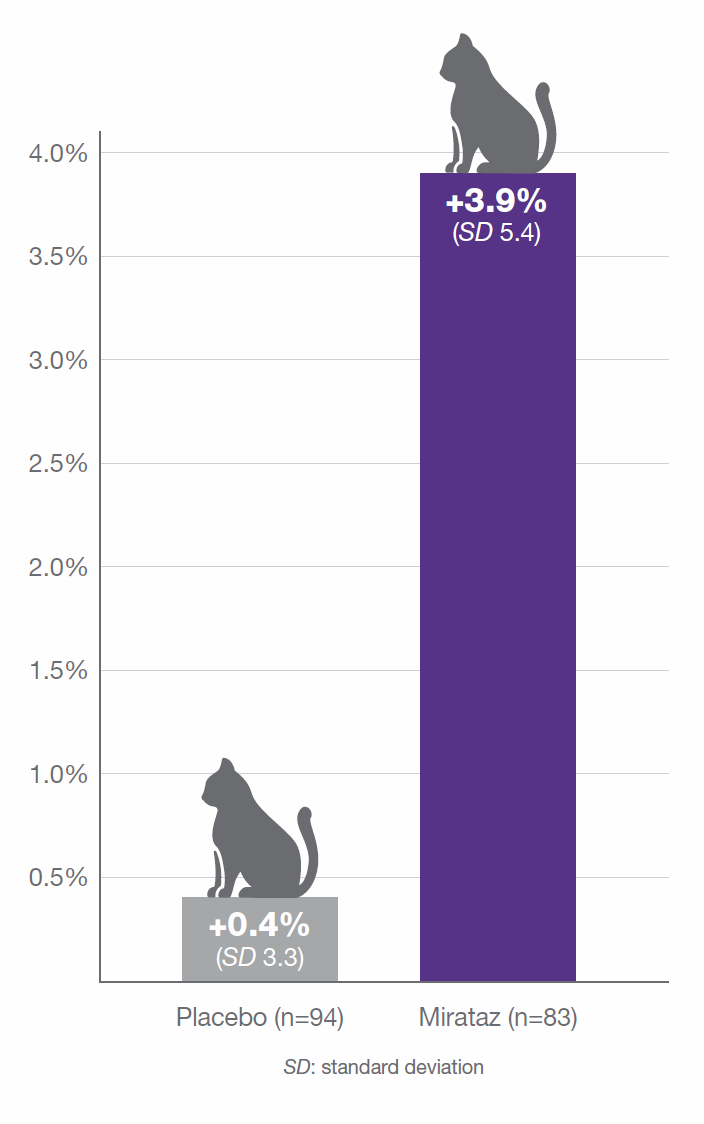
Poole M., Quimby J., et al. (2019) A double blind, placebo-controlled, randomized study to evaluate the weight gain drug, mirtazapine transdermal ointment, in cats with unintended weight loss, Journal of Veterinary Pharmacology and Therapeutics, 42(2) : 179-188
-
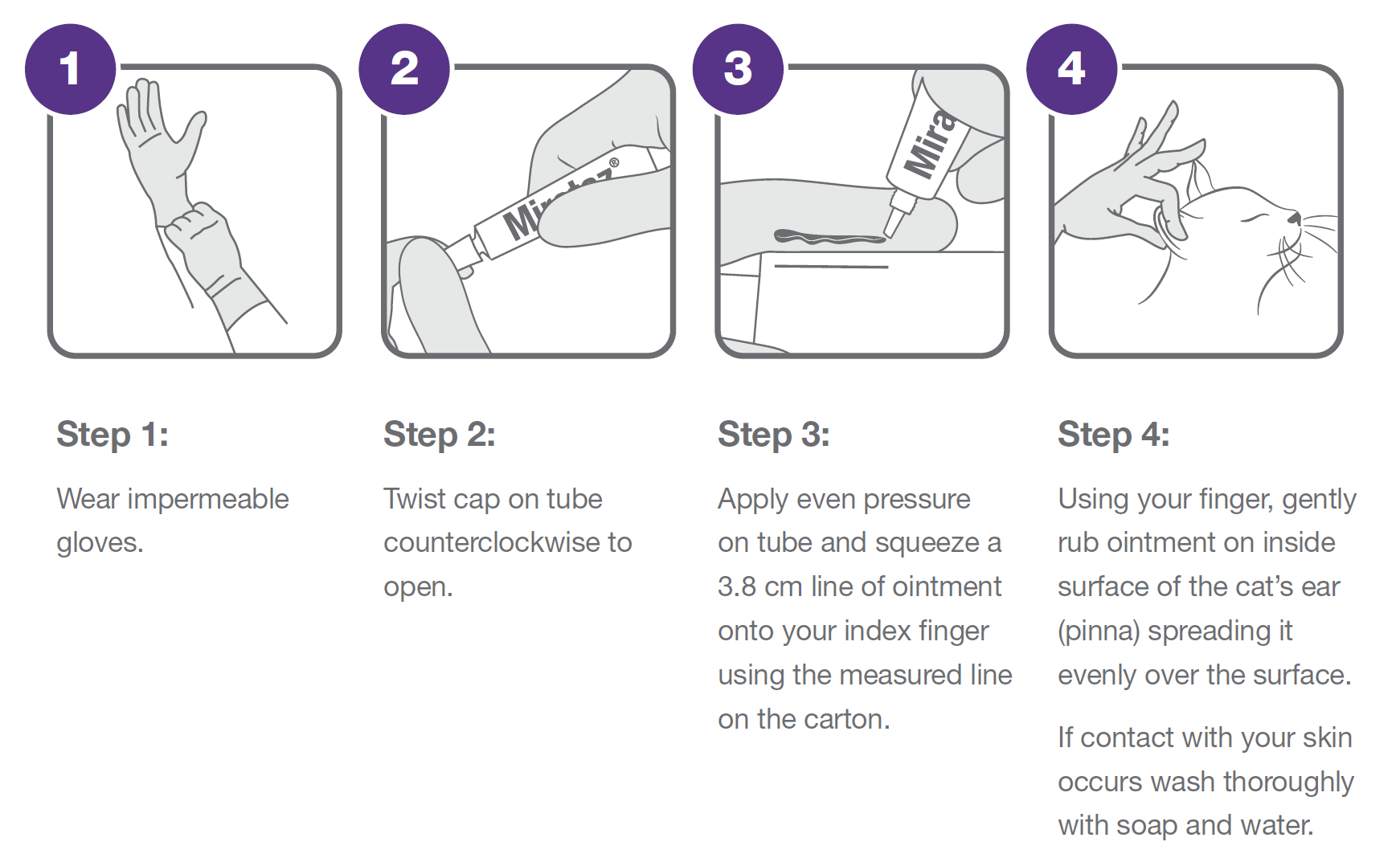
Mirataz 20 mg/g transdermal ointment for cats Package Leaflet updated 10/07/2020
-
Dechra Internal report MZ-0194
-
Dechra Internal report MZ-0193
-
Mason, B., et al. (2019) Double-blind, placebo controlled, randomized study to evaluate the weight gain drug, mirtazapine transdermal ointment, in cats experiencing unintended weight loss: A post-hoc analysis of cats with suspected renal disease, BSAVA Congress Proceedings 424
-
Quimby, J.M., Lunn, K.F., (2013) Mirtazapine as an appetite stimulant and anti-emetic in cats with chronic kidney disease: A masked placebo-controlled crossover clinical trial, The Veterinary Journal, 197: 651-655
-
Ferguson, L.E., McLean, M.K., Bates, J.A., & Quimby, J.A. (2016) Mirtazapine toxicity in cats: retrospective study of 84 cases (2006-2011), Journal of Feline Medicine and Surgery, 18(11): 868-874
-
Benson, K.K., Zajic, L.B., Morgan, P.K., Brown, S.R., et al. (2017) Drug exposure and clinical effect of transdermal mirtazapine in healthy young cats: a pilot study, Journal of Feline Medicine and Surgery, 19(10): 998-1006
-
Cahill, C. (2006 ) Mirtazapine as an Antiemetic. Veterinary Forum, 34-36
-
Quimby, J.M., Gustafson, D.L. and Lunn, K.F. (2011) The pharmacokinetics of mirtazapine in cats with chronic kidney disease and in age‐matched control cats, Journal of Veterinary Internal Medicine, 25(5): 985-989
-
Quimby, J.M., Gustafson, D.L., Samber, B.J. and Lunn, K.F. (2011) Studies on the pharmacokinetics and pharmacodynamics of mirtazapine in healthy young cats, Journal of Veterinary Pharmacology and Therapeutics, 34(4): 388-396
-
Fitzpatrick, R.L., Quimby, J.M., Benson, K.K., et al. (2018) In vivo and in vitro assessment of mirtazapine pharmacokinetics in cats with liver disease, Journal of Veterinary Internal Medicine, 32(6): 1951-1957
-
Buhles, W., Quimby, J.M., Labelle, D., et al. (2018) Single and multiple dose pharmacokinetics of a novel mirtazapine transdermal ointment in cats, Journal of Veterinary Pharmacology and Therapeutics, 41(5): 644-651
-
Calder, P.C. (2003) Long-chain n-3 fatty acids and inflammation: potential application in surgical and trauma patients, Brazilian Journal of Medical Biological Research, 36: 433-446
-
Li, J. et al. (2006) Effects of beta-glucan extracted from Saccharomyces cerevisiae on growth performance, and immunological and somatotrophic responses of pigs challenged with Escheria coli lipopolysaccharide, Journal of Animal Science, 84: 2374-2381.
-
Center, S.A., et al. (2012) Influence of dietary supplementation with L-carnitine on metabolic rate, fatty acid oxidation, body condition, and weight loss in overweight cats, American Journal of Veterinary Research, 73: 1002-1015
*Per protocol population (Cats which completed the entire study through 14 days +/- 3 days) +Safety population (Cats which received at least one dose of Mirataz/Placebo)







 Marine sourced EPA and DHA Omega-3 fatty acids9-10,37
Marine sourced EPA and DHA Omega-3 fatty acids9-10,37 High levels of energy, fat and protein to ensure sufficient nutrients and energy intake even with reduced appetite.
High levels of energy, fat and protein to ensure sufficient nutrients and energy intake even with reduced appetite.


